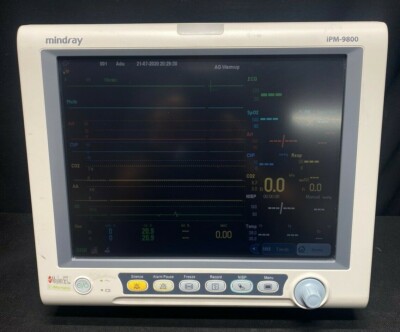
MINDRAY iPM-9800 TOUCH ANESTHESIA CO2 GAS SPO2 NIBP MONITOR.
iPM-9800 patient monitor offers care givers a clear vision of the status of patients of different acuity levels. The portable design delivers monitoring capacity and functionalities in an intuitive way, to suit a wide range of healthcare areas, in and out of hospital. Building on Mindray's heritage in patient monitoring, iPM-9800 integrates highly flexible configurations, a full array of powerful features and versatile data transfer functions, providing great clinical confidence through all clinical areas.
Unfortunately the LCD screen slightly dim & missing handle - but works fine.
BUY IT NOW £399.99!!
DISPATCHED ON DHL EXPRESS DELIVERY (UK NEXT DAY).
WORLDWIDE DISPATCH.
. Touchscreen provides the superior flexibility required for patient management
. Full range parameters enable units to be optimally configured for different departments
. Wired/wireless networking with Hypervisor VI central monitoring system, up to 32 beds in one group
. 2 rechargeable li-ion batteries offer up to 7.5 hours of working time
. Transfer of unit settings using USB flash drive allows fast setup between monitors
. Built-in 3 trace thermal recorder for printing real-time and historical data
. HL7 protocol ready for HIS connection
DISCLAIMER: All medical items are sold in good clean functioning condition unless otherwise stated. It is the buyers responsibility to have the item professionally tested, calibrated or serviced before clinical use on humans or animals.
NOTE: Regardless of the origin of the equipment, documentation provided or identification appearing upon the equipment, the equipment described and offered here is in no way certified for, recommended for, or offered for any specific use. The purchaser agrees that the seller shall not be held responsible or liable for any injuries or damages, whether incidental or consequential, associated in any way with the equipment. The purchaser, by offering on this equipment, indicates their acknowledgment of, and agreement to the terms of this disclaimer.
CE MARKED - This product is in compliance with the essential requirements of Council Directive 93/42/EEC Medical Device Directive, as amended by Council Directive 2007/47/EC, class IIa
NOTE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.