Anti-high frequency surgical unit, defibrillation-proof(special leads are necessary).
Drug concentration calculation and titration table functions.
Review for 71 alarm events of all parameters and 60 arrhythmia alarm events.
AC/DC, built-in rechargeable lithium battery achieve uninterrupted monitoring.
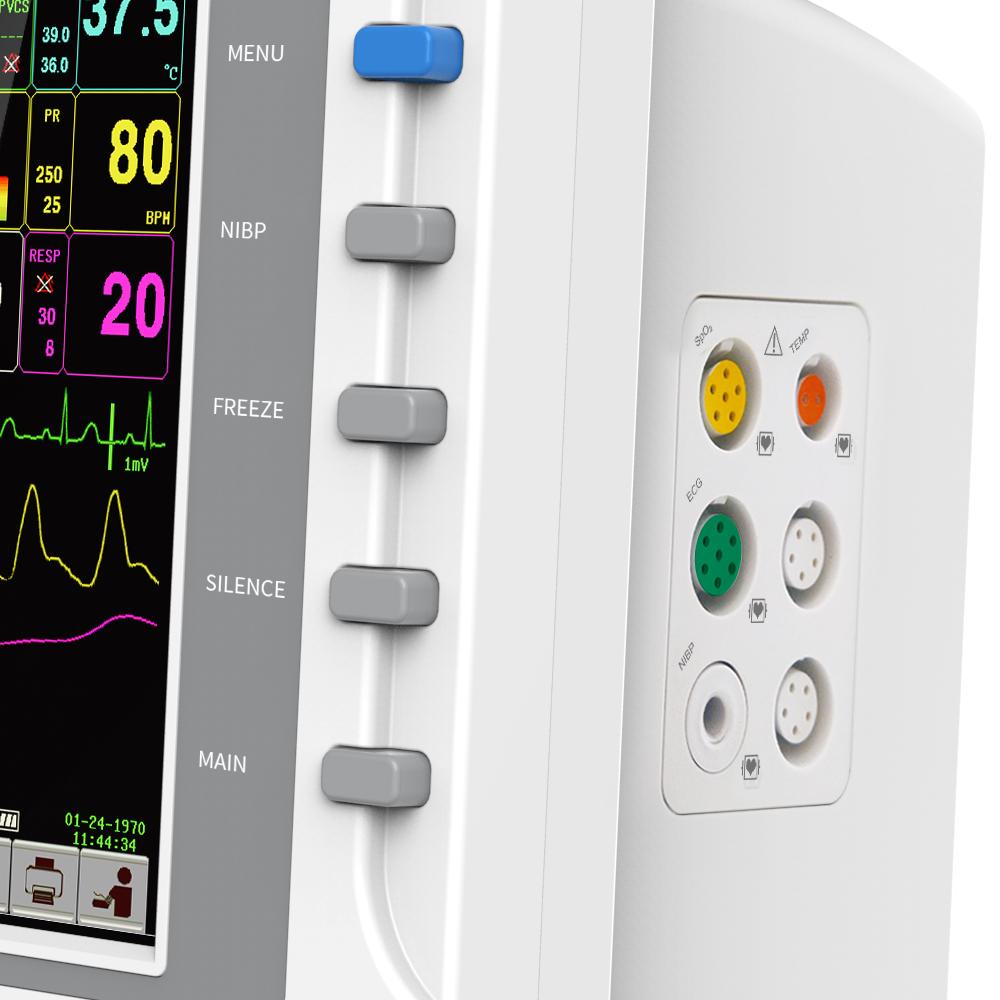
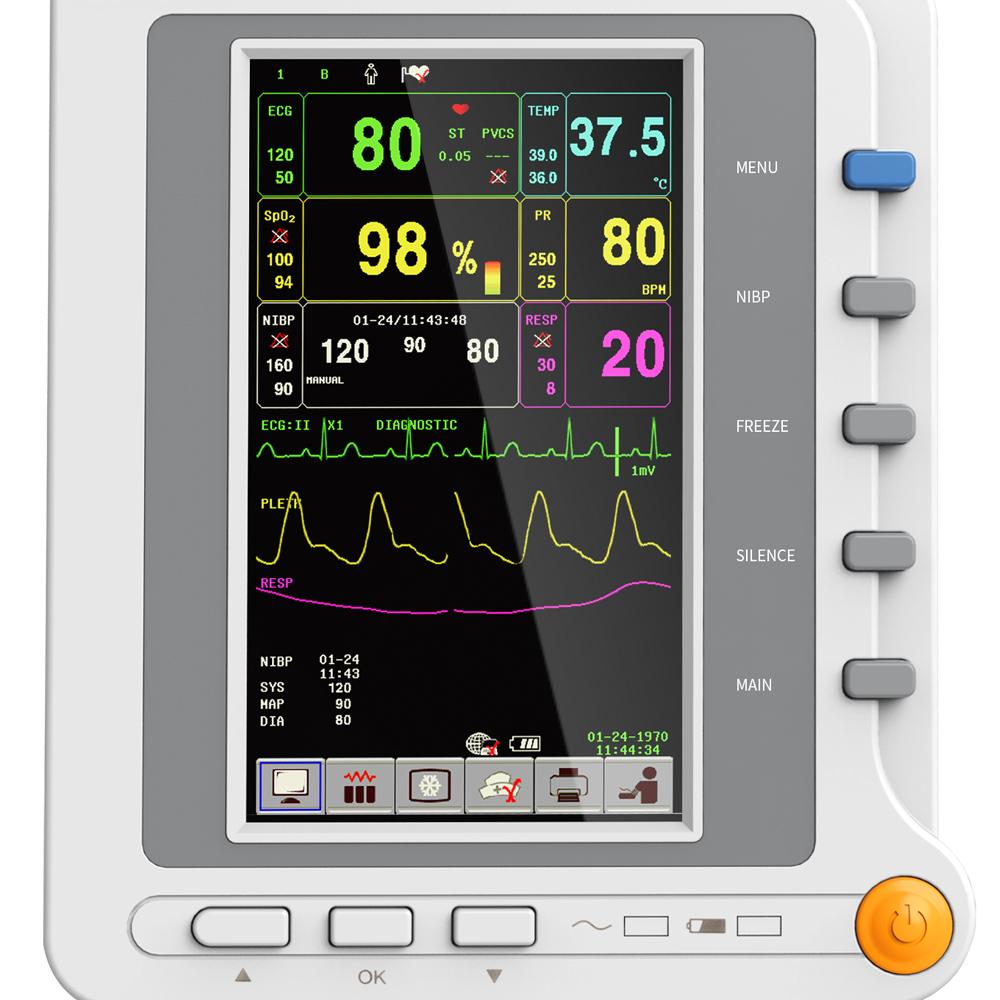
With standard interface, big character interface and view bed, convenient to observe.
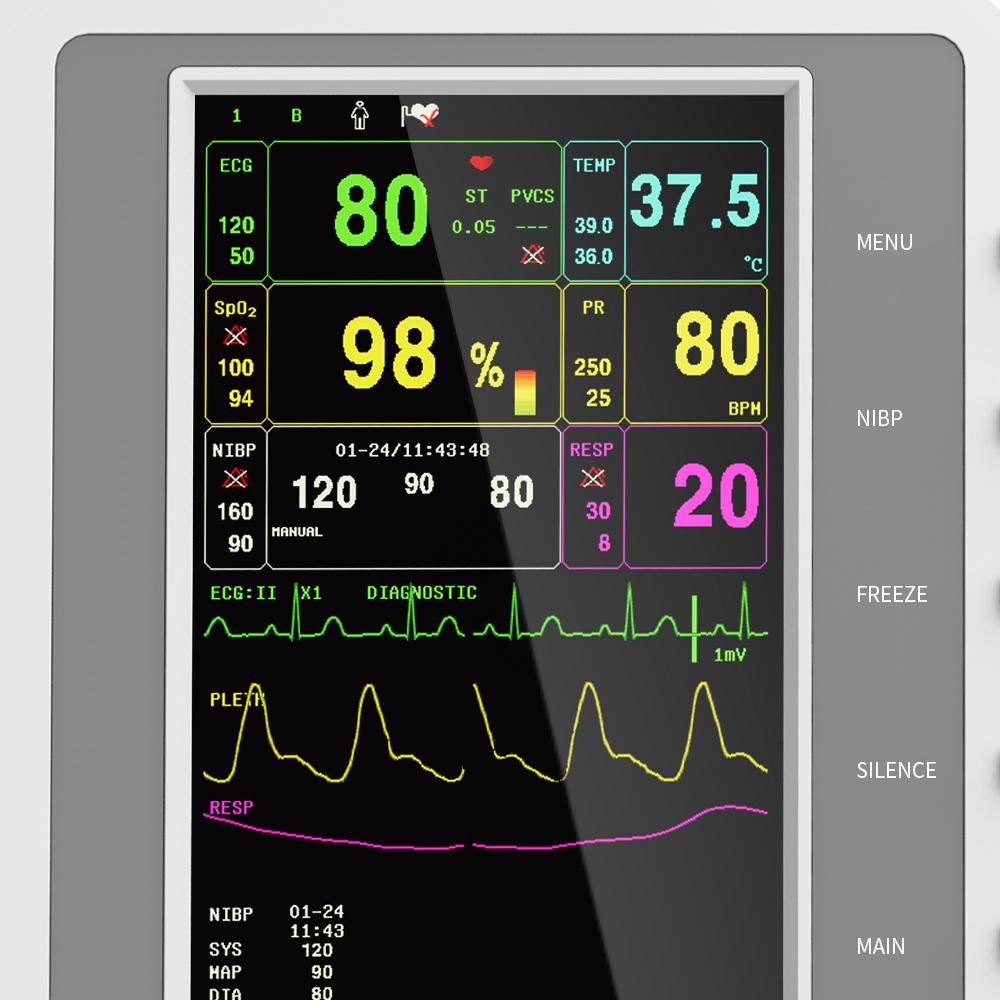
Display 7-lead ECG waveform on one screen.
Waveform, parameter color and location can be set optionally.
Fanless design,quiet,energy-saving and clean,which reduces the possibility of cross-infection.
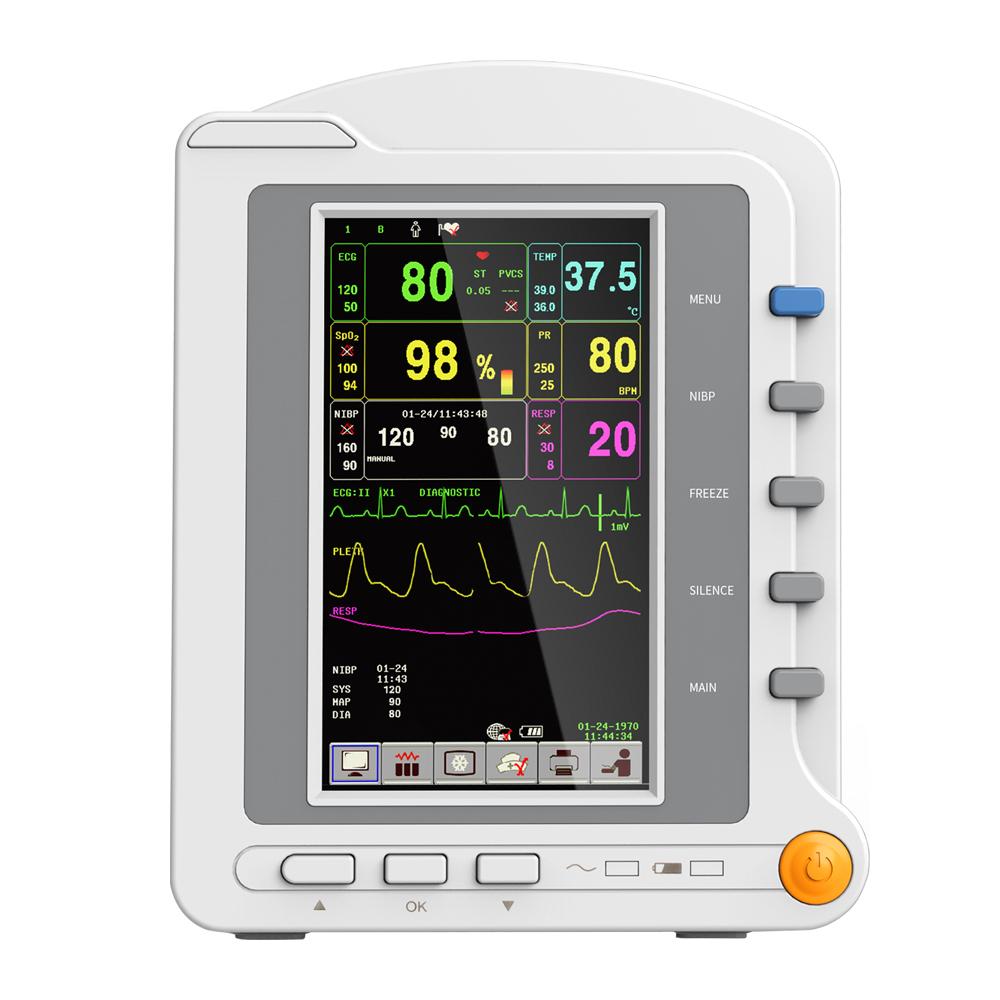
7'' TFT color LCD,multi-language interface(English,Chinese,Russian,Kazakhstan, Czech, Slovak).
Adopt digital SpO2 technology, anti-motion and anti-ambient light interference,and measurement can be performed under the circumstance of weak filling.
NIBP measurement mode:Manual/AUTO/Continuous,storage for 4800-group NIBP data.
All-round monitor for adult, pediatric and neonate.
Connect to Central Monitoring System by 3G, Wi-Fi or wired mode.
This machine can monitor such parameters as ECG, RESP, SpO2, PR, NIBP and TEMP, etc. It integrates parameter measurement module, display and recorder in one device to form a compact and portable equipment.
Parameter:
ECG
Lead mode: 3-lead or 5-lead
Lead selection: I, II, III, aVR, aVL, aVF, V
Waveform: single-channel
Gain: 2.5mm/mV, 5.0mm/mV, 10mm/mV, 20mm/mV
Scan speed: 12.5mm/s, 25 mm/s, 50 mm/s
Measurement and alarm range: 15~350 bpm
Accuracy: ±1 % or ±1 bpm, whichever is greater
Alarm accuracy: ± 2 bpm
Resolution: 1 bpm
ST-segment monitoring:
Measurement and alarm range: -2 mV ~ +2 mV
Accuracy: -0.8 mV~+0.8 mV ±0.04 mV or ±10%, whichever is greater
Other range: unspecified
Arrhythmia analysis: ASYSTOLE, VFIB/VTAC, COUPLET, BIGEMINY, TRIGEMINY, R ON T, VT>2, PVC, TACHY, BRADY, MISSED, PNP, PNC
Pacemaker: yes
RESP
Method: R-F(RA-LL) Impedance
Respiration rate:
Measurement and alarm range: 0~150 rpm
Resolution: 1 rpm
Measurement accuracy: ±2 rpm
Alarm accuracy: ±3 rpm
Apnea alarm: 10~40s
Scan speed: 12.5 mm/s, 25 mm/s
NIBP
Method: Oscillometry
Mode: Manual/AUTO/Continuous
Measurement interval in AUTO mode: 1/2/3/4/5/10/15/30/60/90/120/240/480 minutes
Measurement period in Continuous mode: 5 minutes
Measurement and alarm range: 10 ~ 270 mmHg
Resolution: 1 mmHg
Cuff pressure accuracy: ±3 mmHg
Measurement accuracy:
Maximal mean deviation: ±5 mmHg
Maximal standard deviation: 8 mmHg
Over-pressure protection:
Adult mode: 297±3 mmHg
Pediatric mode: 240±3 mmHg
Neonatal mode: 147±3 mmHg
SpO2
Measurement and alarm range: 0 ~ 100%
Resolution: 1%
Measurement accuracy: 70%~100%: ±2%;
0%~69%: unspecified
PR
Measurement and alarm range: 30 ~ 250 bpm
Measurement accuracy: ±2 bpm or ±2%, whichever is greater
TEMP
Channel: single-channel
Measurement and alarm range: 0 ~ 50℃
Resolution: 0.1 ℃
Accuracy: ±0.1 ℃
Power supply
Adapter: 9 (V) DC
AC: 100 ~ 240 (V), 50/60 (Hz)
Maximal input power: 150VA
Safety classification: Class I, type CF defibrillation-proof applied part
Package List:
1×Patient monitor
1×Adult fingertip SpO2 probe
1×SpO2 probe extension cable
1×Adult NIBP cuff
1×NIBP extension tube
1×ECG lead cable
1×ECG electrode
1×Temperature probe
1×Power cord
1×Power adapter
1×User Manual