Description:
This medical endoscope and arthroscope ent connector is made with high-quality stainless steel and utilizes Germany optic fiber and glass for superior image quality. The direction index sapphire lens cover prevents abrasion and ensures durability. This device can be sterilized by gas or soaking for safe use. Perfect for medical professionals seeking a reliable and high-performing tool for various procedures.
Specification
Size:?4mmX175 mm
Degree: 30°
Packing List
Arthroscope ?4mmX175 mm ?: 1 each

Shipping Policy
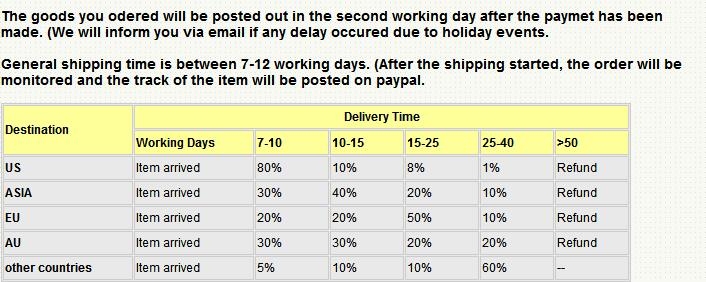
- Payment
- We accpet PAYPAL payment, it's easy and safe.If you would like to pay by other method please contact me.
- If you buy more than one item.payment should be paid completely within 7 days.
- Your items will be shipped as a gift to avoid the customs tax problems. if any additional charges generated for customs clearance must be borne by customers.Any question please contact me.
- Import duties, taxes and charges are not included in the item price or shipping charges. These charges are the buyer’s responsibility. Please check with your country’s customs office to determine what these additional costs will be prior to bidding / buying.These charges are normally collected by the delivering freight (shipping) company or when you pick the item up – do not confuse them for additional shipping charges.
Return Policy
If you receive a defective item which you want to return, please contact us within 2 days from the day you receive the item. All return items must be returned with its original packaging and accessories. If we confirm the damage happened during the shipment, we will bear the shipping cost for return.
We will refund the money to you when we get the return items. Or replace item for you free of charge.
About Warranty
We guarantees new equipment other than accessories to be free from defects in workmanship and materials for a period of twelve months (six months for spare parts) from the date of shipment under normal use and service. Our company's obligation under this warranty is limited to repairing, at our company's option, any part which upon our company's examination proves defective.
IMPORTANT INFORMATION ON OUR POLICY AND FEEDBACK
- Please leave me FIVE STAR POSITIVE feedback if you are satisfied with our items.
- We try to be fair and accurate with all of our product listings and descriptions. Please read each auction, bid, and sale carefully.
- We will appreciate it if you please email us before leaving negative feedback. If there is a problem, we can work together to resolve it.
We strive to always be extremely fair and always try to work with reasonable customers. - Negative feedback doesn't go away and should only be left as a last resort! We strive for positive feedback, harmonious relations and even friendships.
We will always make every attempt possible to resolve issues if we made a mistake.(It happens). Please do not assume a mistake is intentional.
Let us know if our service could be better!!
FDA Disclaimer:
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item. (Lucas-China-Beijing-86-15346852147)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892
Ultrasound ,Ultrasonic Treatment Device is certified with the US FDA 510(k) Number:K161892
Our working time:
Monday - Friday?? 9:00 AM - 5:30PM (Beijing)?
Any questions, please contact us through eBay message or e-mail, We will reply within 1-2 business days (public holidays not included).